The effect of the poly(vinyl phenol) sublayer on the melting behavior of poly(butylene adipate) crystals
Quan Li, Jiandong Zhou, Liguo Chai, Jamil Memon, Zhongjie Ren, Huihui Li, Xiaoli Sun and Shouke Yan
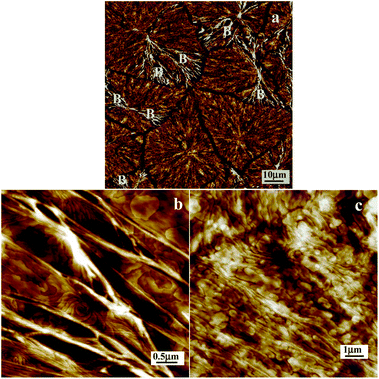
Abstract : The crystallization and melting behaviors of PBA thin films with different thicknesses placed on Si wafers and PVPh surfaces under varied conditions were studied by using grazing incident X-ray diffraction (GIXD) and infrared reflection-absorption spectroscopy (IR-RAS). The results show that crystallization of PBA during the solvent evaporation process on either Si wafer or PVPh surfaces produces always β-form crystals regardless of the film thickness. However, the melting behavior of the β-PBA crystals on the PVPh surface is quite different from those on a Si wafer. On the Si wafer, the melting of β-PBA crystals in films with thicknesses ranging from 39 to 293 nm is not altered obviously. The β-to-α phase transition always takes place during the heating process before melting. By contrast, on the PVPh sublayer, the melting of the β-PBA crystals depends on the thicknesses of both PBA and PVPh layers. A thinner PBA layer and a thicker PVPh layer favor a direct melting of the β-PBA crystals without the occurrence of β-to-α phase transition. The phase transition temperature of the PBA film with the same thickness reduces with the thickening of the PVPh layer. IR-RAS results indicate that the intermolecular hydrogen bonds increase with temperature and thickening of PVPh sublayers. The reduced phase transition temperature can be attributed to the increasing hydrogen bonds formed at the interface of PVPh and PBA. The surface property of PVPh films is investigated conveniently through monitoring the formation of hydrogen bonds between PVPh and PBA at the interface. The formation of hydrogen bonds at temperatures which are much lower than the glass transition temperature of PVPh suggests that the segmental mobility of PVPh molecular chains evolves in the glassy state. It is the higher segmental mobility of PVPh in the thicker film that alters the phase transition behavior of the PBA layer.