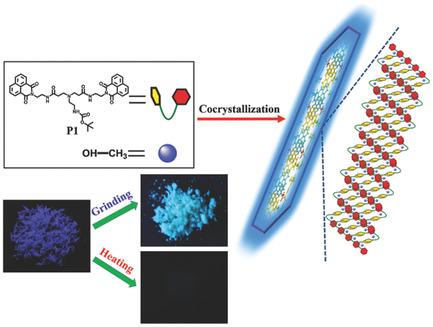
Organic luminescent materials with the ability to reversibly switch the luminescence when subjected to external stimuli have attracted considerable interest in recent years. However, the examples of luminescent materials that exhibit multiresponsive properties are rarely reported. In this work, a new stimuli‐responsive dye P1 is designed and synthesized with two identical chromophores of naphthalimide, one at each side of an amidoamine‐based spacer. This amide‐rich molecule offers many possibilities for forming intra‐ and intermolecular hydrogen bond interactions. Particularly, P1 has an intrinsic property of cocrystallizing with methanol. Compared with the pristine P1 sample, the as‐prepared two‐component cocrystalline material displays an exceptive deep‐blue emission, which is extremely rare among naphthalimide‐based molecules in the solid state. Furthermore, the target material exhibits an obvious mechanochromic fluorescent behavior and a large spectral shift under force stimuli. On the other hand, the cocrystalline material shows an unusual “turn off” thermochromic luminescence accompanied by solvent evaporation. Moreover, using external stimuli to reversibly manipulate fluorescent quantum yields is rarely reported to date. The results demonstrate the feasibility of a new design strategy for solid‐state luminescence switching materials: the incorporation of solvents into organic compounds by cocrystallization to obtain a crystalline state luminescence system.