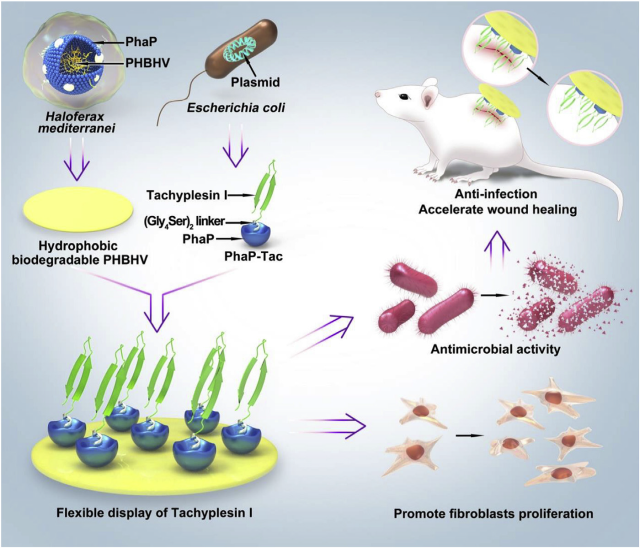
Implants decorated with antimicrobial peptides (AMPs) can prevent infection and reduce the risk of creating antibiotic resistance. Yet the restricted mobility of surficial AMP often compromises its activity. Here, we report a simple but effective strategy to allow a more flexible display of AMP on the biomaterial surface and demonstrate its efficacy for wound healing. The AMP, tachyplesin I (Tac), is tagged with the polyhydroxyalkanoate-granule-associated protein (PhaP) and immobilized on haloarchaea-produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBHV) via hydrophobic interaction. The PhaP-Tac coating effectively inhibits the growth of both Gram-negative and Gram-positive bacteria. It also increases the surface hydrophilicity to improve fibroblast proliferation in vitro, and accelerates wound healing by decreasing bacterial counts to below 105 CFU per gram of tissue in a deep-wound mouse model in vivo. Taken together, these findings demonstrate an effective strategy to realize the full potential of AMPs in imparting implants with an anti-microbial activity that is localized and potent.