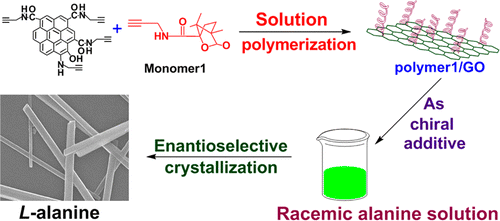
This article reports an original, versatile strategy to chirally functionalize graphene oxide (GO) with optically active helical-substituted polyacetylene. GO was first converted into alkynyl-GO containing polymerizable –C≡C moieties, which took part in the polymerization of another chiral acetylenic monomer, yielding the expected GO hybrid covalently grafted with chiral helical polyacetylene chains. Tran**ission electron microscopy, atomic force microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, and thermogravimetric ****yses verified the successful attachment of substituted polyacetylene chains on GO by covalent chemical bonding. Moreover, circular dichroi** effects and UV−vis absorption demonstrated that the GO hybrid possessed fascinating optical activity. It also largely improved the dispersibility of GO in tetrahydrofuran. The GO-derived hybrid was further used as a chiral inducer toward enantioselective crystallization of alanine enantiomers. L-Alanine was preferably induced to crystallize, forming rodlike crystals.