Extended “adsorption-insertion” model: A new insight into the sodium storage mechani** of hard carbons --- Advanced Energy Materials, 2019, 9, 1901351.
Ning Sun, Zhaoruxin Guan, Yuwen Liu, Yuliang Cao, Qizhen Zhu, Huan Liu, Zhaoxiang Wang, Peng Zhang and Bin Xu*
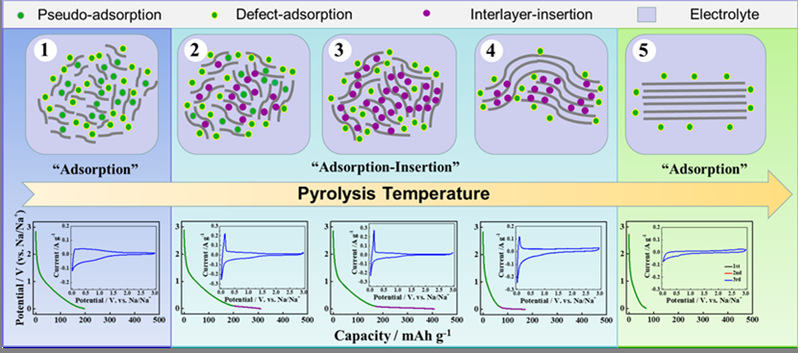
Hard carbons (HCs) are promising anodes of sodium-ion batteries (SIBs) due to their high capacity, abundance, and low cost. However, the sodium storage mechani** of HCs remains unclear with no consensus in the literature. Here, based on the correlation between the microstructure and Na storage behavior of HCs synthesized over a wide pyrolysis temperature range of 600-2500 °C, an extended “adsorption-insertion” sodium storage mechani** is proposed. The microstructure of HCs can be divided into three types with different sodium storage mechani**s. The highly disordered carbon, with d002 (above 0.40 nm) large enough for sodium ions to freely transfer in, has a “pseudo-adsorption” sodium storage mechani**, contributing to sloping capacity above 0.1 V, together with other conventional “defects” (pores, edges, heteroatoms, etc.). The pseudo-graphitic carbon (d-spacing in 0.36-0.40 nm) contributes to the low-potential (<0.1 V) plateau capacity through “interlayer insertion” mechani**, with a theoretical capacity of 279 mAh g-1 for NaC8 formation. The graphite-like carbon with d002 below 0.36 nm is inaccessible for sodium ion insertion. The extended “adsorption–insertion” model can accurately explain the dependence of the sodium storage behavior of HCs with different microstructures on the pyrolysis temperature and provides new insight into the design of HC anodes for SIBs.