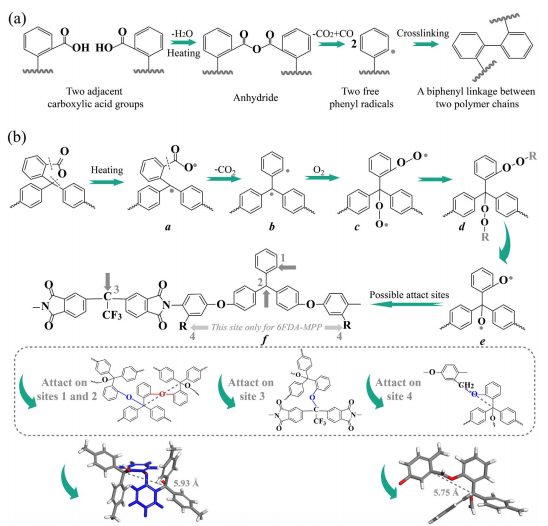
A proper increase of d-spacing after crosslinking is an effective strategy to enhance gas separation performance and anti-plasticization property of polymeric membranes. One way to achieve high chain distance is to design and prepare polymer precursors that have large side groups with crosslinkable functionality. Our previous research proved that the decarboxylation crosslinked polyimides connected by a biphenyl group showed enlarged d-spacing. However, the 100% crosslinking temperature is 425-450 degrees C that is higher than the glass transition temperature (T (g)) of most polymers and may cause problem for preparing asymmetric crosslink membranes. Here we proposed a thermal oxidative crosslinking method which had a lower crosslinking temperature than the decarboxylation crosslinking method. Specifically, we synthesized two phenolphthalein-based cardo diamines, MPP and PP, with or without CH3 substituted groups and prepared the 6FDA-MPP and 6FDA-PP polyimides. The lactone ring in the cardo moiety decomposed and the crosslinking reaction took place by heating the polymer in an air purge atmosphere. A100% conversion of the crosslinking reaction was obtained at 400 degrees C. In addition, the crosslinked polyimides showed increased d-spacing, high CO2 permeability and CO2/CH4 selectivity than before. The best result for pure gas test was achieved for 6FDA-MPP-400, which had a CO2 permeability of 193.8 Barrer with a CO2/CH4 ideal selectivity of 39.2. No plasticization was observed for the 6FDA-MPP-400 and 6FDA-PP-400 polyimides at a CO2 pressure up to 450 psia. In addition, both 6FDA-MPP-400 and 6FDA-PP-400 showed excellent mixed gas selectivity of 35 at a partial CO2 pressure of 147 psia.